Atomic Mass of Nitrogen
Option A is incorrect. Note that each element may contain more isotopes therefore this resulting atomic mass is calculated from.

Nitrogen Definition Symbol Uses Properties Atomic Number Facts Nitrogen Electron Configuration Hydrogen Bond
The atomic mass of each isotope number of protons number of neutrons.

. Up to 24 cash back The atomic mass of nitrogen is 14007 AMU. Atomic mass of Nitrogen is 140067 u. Nitrogen molecular weight.
See also our theoretical yield calculator for chemical reactions probably your next stop to finish the. The symbol for nitrogen is N. Chemistry Matter Atomic Mass.
A Nitrogen atom with 7 protons and 8 neutrons has a mass number of 1 5 a m u. The units of measurement for this is gramsmole or simply gmol. Convert grams Nitrogen to moles or moles Nitrogen to grams.
Natural nitrogen 7 N consists of two stable isotopes. The atomic mass of nitrogen is 14. Chemical element Nitrogen information from authoritative sources.
The molar mass of N2 Molecular Nitrogen is. For example nitrogen has an atomic mass of 14 but. Nitrogen has an atomic mass of 1401 amu or 1401 gmol.
We know that the atomic number of nitrogen is 7 and the atomic mass number is about 14. Molar mass of N 140067 gmol. Find step-by-step Chemistry solutions and your answer to the following textbook question.
The atomic weight of boron. The formula weight is simply the weight in atomic mass units of. The unit AMU represents atomic mass unit.
Atomic Mass of Nitrogen. Therefore a nitrogen atom has seven. Look up properties history uses and more.
The stable isotopes of nitrogen are 14N and 15N. The unit of atomic mass is u unified mass Atomic mass of Nitrogen. Each element has its own atomic mass.
What is the atomic mass of nitrogen. The difference between atomic mass and atomic number is important because it determines how an atom is grouped in the periodic table. Instead the atomic mass is equal to the number grams of the element in one mole of atoms of the element.
However on the periodic table the atomic mass for Nitrogen is 1 4. The first is far more prevalent accounting for 99634 percent of natural nitrogen. Now for the right answer to the above question.
Another unit for atomic mass is the dalton Da. The atomic masses of the two stable isotopes of. 1 Answer anor277 Sep 13 2016 Surely you must have access to a Periodic Table.
Number of neutrons in nitrogen. Neutron 14 7 7. All of these radioisotopes are short-lived the longest-lived being nitrogen-13 with a half-life of.
The vast majority 996 of naturally occurring nitrogen is nitrogen-14 with the remainder being nitrogen-15Fourteen radioisotopes are also known with atomic masses ranging from 10 to 25 along with one nuclear isomer 11m N. The average atomic mass of nitrogen is 140067. How is this possible.

Nitrogen Is A Chemical Element With The Symbol N And Atomic Number 7 Dinitrogen N2 Forms About 78 Chemistry Education Chemistry Experiments Chemistry Class

Nitrogen Chemical Properties Uses Atomic Number Periodic Table Chemistry Basics Chemistry Nitrogen
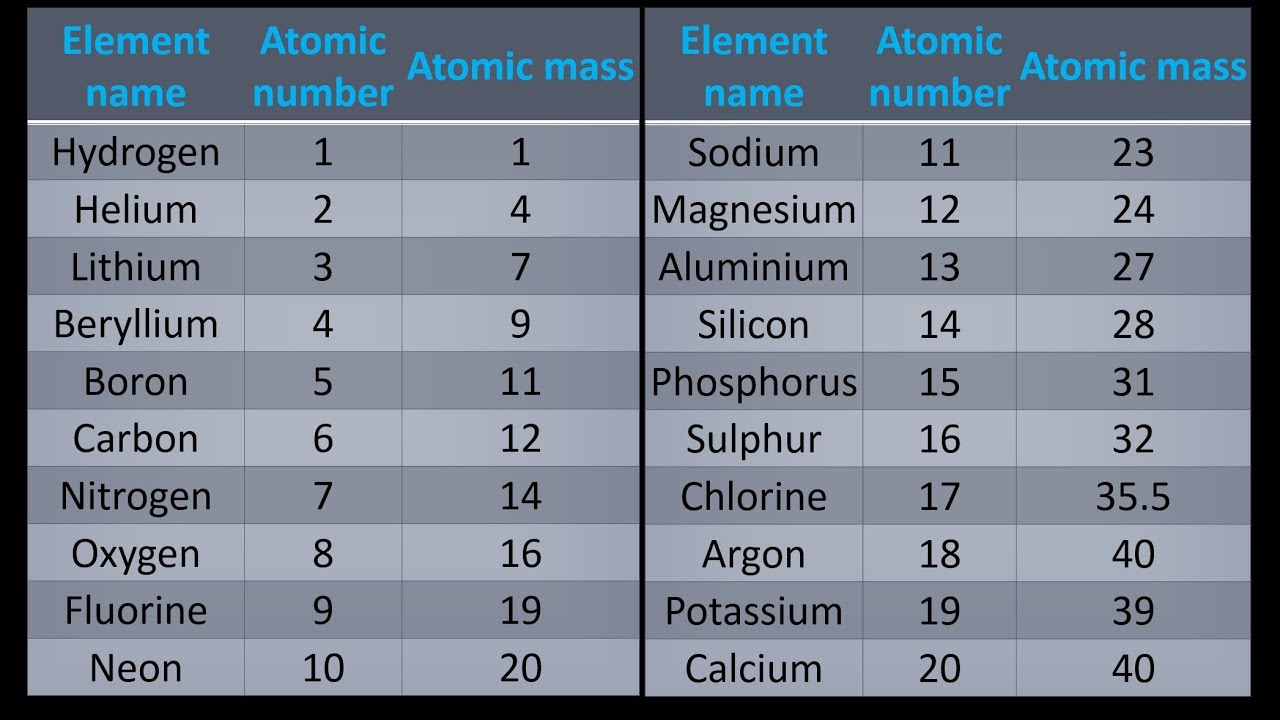
A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Table Youtube Chemistry Lessons Element Chemistry Chemistry Basics

No comments for "Atomic Mass of Nitrogen"
Post a Comment